It is an age old dream of medicine: if arbitrary kinds of tissue could be produced artificially from stem cells, then injuries could be healed with the body’s own cells, and one day it might even be possible to produce artificial organs. However, it is difficult to get cells into the desired shape. The methods that have existed so far can be divided into two fundamentally different categories: Either one first creates small tissue building blocks, such as round cell agglomerates or flat cell sheets, and then assembles them, or one initially creates a fine, porous scaffold that is then cultivated with cells. Both approaches have advantages and disadvantages.
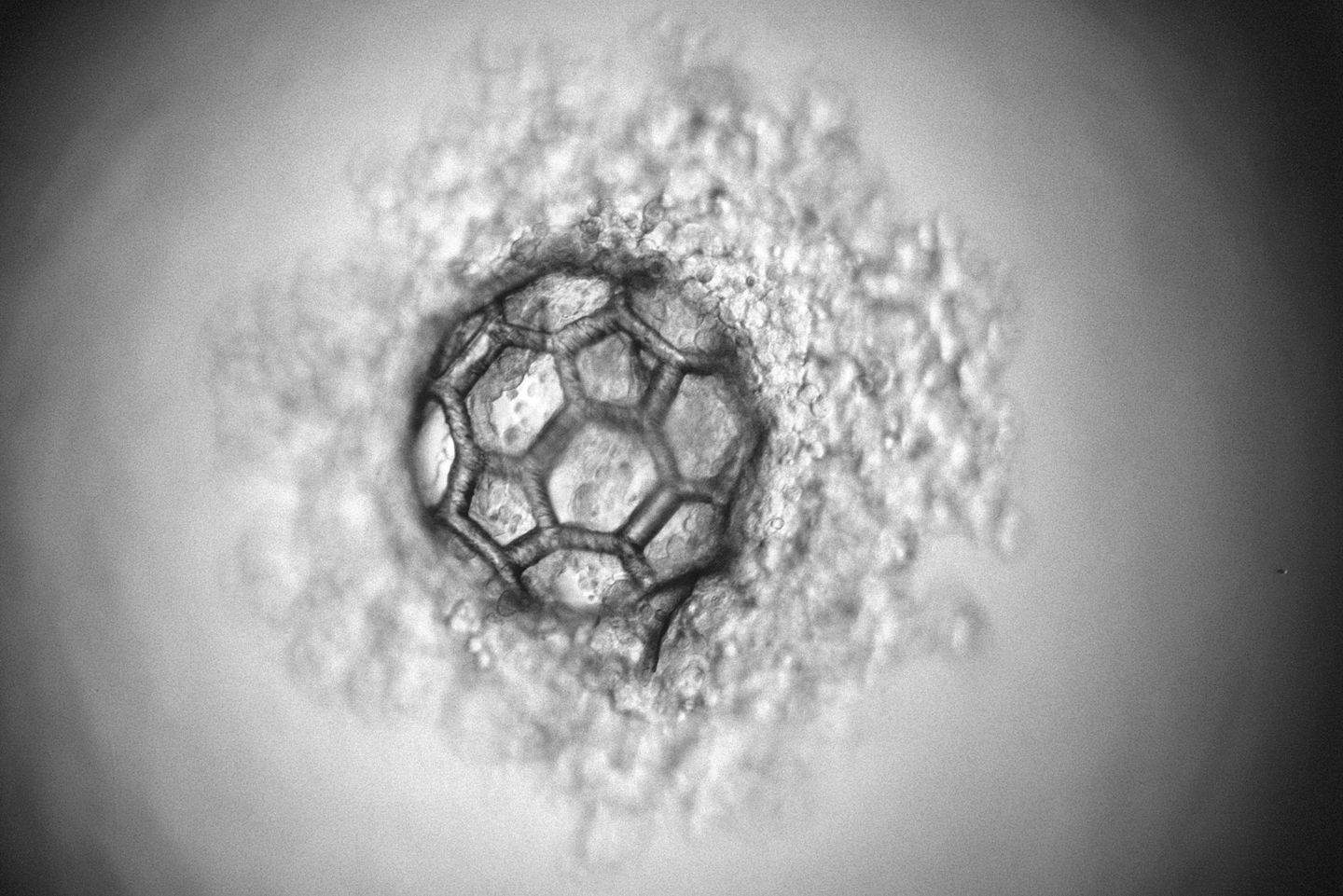
At TU Wien (Vienna), a third approach has now been developed: Using a special laser-based 3D printing technique, micro-scaffolds with a diameter of less than a third of a millimetre can be produced, which can accommodate thousands of cells. In this way, a high cell density is present from the start, but one still has the flexibility adapt the shape and mechanical properties of the structure.
With scaffold or without?
“The scaffold-based approaches that have been developed so far have great advantages: If you first make a porous scaffold, you can precisely define its mechanical properties,” says Dr Olivier Guillaume, lead author of the current study, who is researching at TU Wien in the team of Prof Aleksandr Ovsianikov at the Institute of Materials Science and Technology. “The scaffold can be soft or hard as needed, it consists of biocompatible materials that are degraded in the body. They can even be equipped with special biomolecules that promote tissue formation.”
The downside, however, is that it is difficult to quickly and completely populate such a scaffold with cells. A lot of manual work is still needed here today, even though research is already being done on automated processes. Especially with large scaffolds, it takes a long time for the cells to migrate into the interior of the structure; often the cell density remains very low and inhomogeneous.
The situation is completely different if no such scaffold is used. It is also possible to simply grow small cell agglomerates, which are then joined together in the desired shape so that they eventually merge. With this technique, the number of cells is large from the start, but there are hardly any possibilities to intervene in the process. For example, it can happen that the cell spheres change their size or shape and the tissue ends up with different properties than desired.
Living cells meet high-resolution 3D printing process
“We have now succeeded in combining the advantages of both approaches – using an extremely high-resolution 3D printing method that we have been researching here at TU Wien for years,” says Prof. Aleksandr Ovsianikov.
This technique, two-photon polymerisation, uses a light-sensitive material that is cured with a laser beam exactly at the desired positions. In this way, structures can be produced with an accuracy in the range of less than one micrometre.
This laser method is now used to create filigree, highly porous scaffolds with a diameter of just under a third of a millimetre. The design of these micro-scaffolds enables the rapid generation of cell agglomerates inside. At the same time, the cells are protected from external mechanical damage, similar to the way a rally driver is protected by a race car roll cage.
“These cell-filled scaffolds are relatively easy to handle and can coalesce,” explains Aleksandr Ovsianikov. “When many of them are brought into direct contact, it is possible to create large tissue constructs with a high initial cell density in a short time. Still, we can control the mechanical properties of the structure well.”
Cartilage and bone as first target tissues
The underlying concept of this novel tissue engineering strategy was already presented in detail by the research group in 2018. Now, for the first time, it has been possible to show that this method actually works: “We were able to show that the method actually delivers the benefits we were hoping for,” says Aleksandr Ovsianikov. “We used stem cells for our experiments, which can be induced to produce either cartilage or bone tissue. We were able to show that the cells from neighbouring scaffold units do indeed merge and actually form a single tissue. In doing so, the structure retains its shape. In the future, these scaffold units could even be made injectable for use in minimally invasive surgery.”
This work was conducted in the framework of the ERC frontier research project THIRST (ERC Consolidator Grant agreement ID: 772464).
Original publication
Additional information: